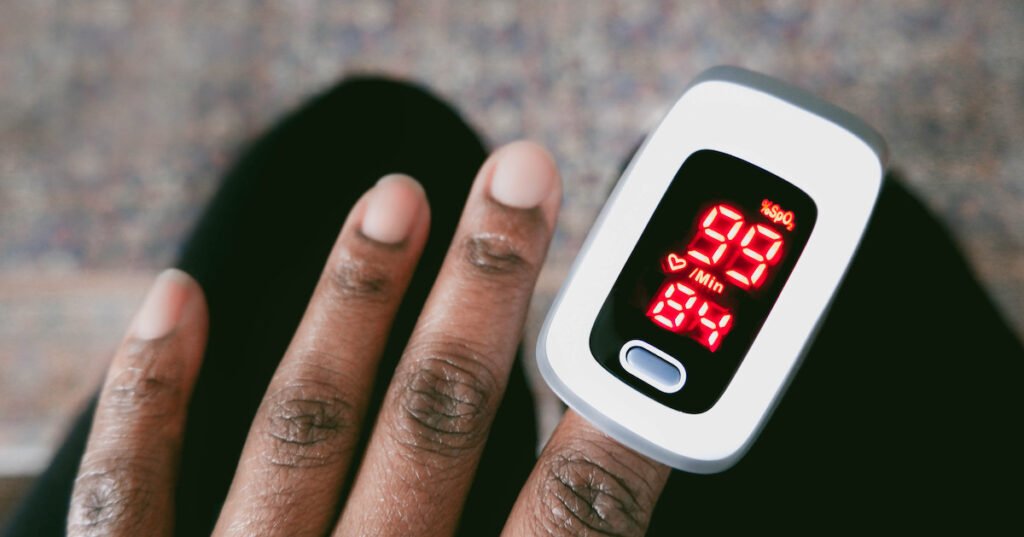
The U.S. Food and Drug Administration is seeking people feedback on recent recommendations for nonclinical and scientific achievement tests to help intraday applications for amplitude oximeters for medical purposes. These include products that calculate the blood pressure and gas content. WHY IS IT MATTERNS During the COVID-19 crisis, medical scientists and clinicians found proof that pulse oximeters did not often deliver accurate readings for darker skin tones. The FDA issued a notice about potential dangers of error under certain conditions in 2023 and said it became conscious that pulse oximeters might be less precise in people with dark skin color. The organization therefore held open hearings last year to review its current pulse oximetry recommendations. The new proposed update, which is available on its website, strengthens recommendations for gathering real-world and laboratory scientific data to assess the reliability of the device’s performance across the range of body pigmentations. Using both subjective and objective methods, the new proposed release standardizes evaluations of signal oximeter products used in care delivery. The advice, Pulse Oximeters for Medical Purposes – Non-Clinical and Medical Performance Testing, Labeling, and Premarket Submission Recommendations, suggests novel performance data in product naming and only applies to specific pulse oximeters, which are mainly used for medical purposes in hospitals or physicians ‘ agencies, according to FDA in a statement on Monday. Some pulse oximeters that are currently on the market may meet the updated performance requirements, the organization explained. There are no significant hardware or software changes required. The FDA stated that the agency would provide an expeditious review within 30 days to ensure the public has prompt access to safe and accurate pulse oximeters. If a sponsor of such a device only submits “updated labeling to reflect the collection of clinical data that demonstrates comparable performance across skin pigmentations without device modifications,” the FDA would not apply to general wellness products or sporting/aviation products that the FDA does not review or evaluate before being made available to the public. Before the agency begins working on the final version of the guidance, comments made by March 10 will be taken into account, it said. According to a study published in the New England Journal of Medicine in 2020, Black patients were three times more likely than white patients to have occult hypoxemia when compared to measurements of arterial oxygen saturation and pulse oximetry. The FDA’s medical device regulation of pulse oximeters has been the subject of Johns Hopkins School of Medicine’s attention. It has criticized how the company handled its 510 ( k ) review process for bringing these products to the medical, consumer, and other industry markets, as well as criticizing the agency’s response time. In response, a FDA spokesperson stated in July that it was still engaging stakeholders and gathering ideas from clinical researchers to inform the update. Prior to the FDA’s Center for Devices and Radiological Health Guidance Agenda, it had stated that it would publish updates in FY 2024 and that it would prioritize revisiting the pulse ox guidance. The healthcare organization called it a striking illustration of how systemic racism can affect healthcare and population health outcomes in a lawsuit filed to stop the sale of flawed pulse oximeters by the Roots Community Health Center in Oakland, California. According to the American Heart Association, heart disease death rates are also highest among Black Americans, and racial bias inherent in light-sensing and other technologies could be potential links to the demographic’s elevated risks. In October, Medtronic announced that it had been barred from the federal lawsuit against GE Healthcare, CVS, Walgreens, GE Healthcare, and numerous other medical technology companies. According to a company spokesman, Medtronic has invested in improving the diversity of its clinical studies, increasing its investment in improving the use of pulse oximetry, and investing in new technology over the past two years. We’re pleased that Medtronic, which has a dominant share of the pulse oximeter market, has chosen to prioritize patient safety by sending California hospital customers a warning about potential flawed readings for those with darker skin pigmentation, Roots stated in an email following the announcement. ON THE RECORD” This draft guidance is aligned with the FDA’s broader commitment to helping facilitate the development of high-quality, safe, and effective medical devices”, said Dr. Michelle Tarver, FDA’s CDRH director, in a statement. Our draft recommendations are based on the best scientific research that can address concerns about disparate pulse oximeter performance based on a person’s skin pigmentation. Andrea Fox is senior editor of Healthcare IT News.
Email: afox@himss.org
Healthcare IT News is a HIMSS Media publication.
FDA improvements advice on medical pulse oximeters.